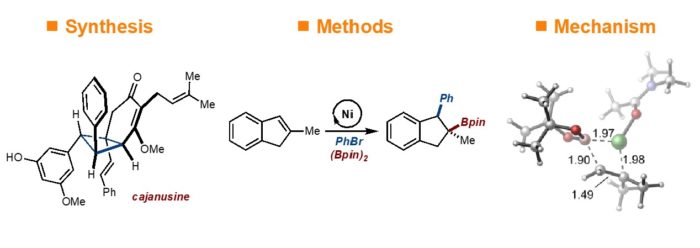
The discovery and development of small organic molecules as new medicines is one of the most important objectives in modern biomedical research. Central to fueling the pipeline of new medicines is the chemical synthesis of novel small molecules. Stereochemically and topologically complex molecules are particularly challenging to prepare in useful quantities, even with state-of-the-art chemical reactions. Therefore, the discovery and development of novel and widely applicable stereoselective chemical reactions stands at the forefront of modern organic chemistry research.
Methods Development:
Alkenes constitute an appealing class of starting materials for chemical synthesis because of their wide availability and ease of synthesis. Difunctionalization reactions are particularly important as two new bonds and two new stereocenters can be generated in a single operation thus allowing for the rapid buildup of molecular complexity. Along this theme, we are making innovations in transition-metal catalyzed reactions and cycloaddition processes.
Representative Papers:
1) “Nickel-Catalyzed Arylboration of Alkenylarenes: Synthesis of Boron-Substituted Quaternary Carbons and Regiodivergent Reactions” Liang-An Chen, Alan R. Lear, Dr. Pin Gao, and M. Kevin Brown* Angew. Chem. Int. Ed. 2019, 58, 10956.
2) “Synthesis of Biheteroarylalkanes by Heteroarylboration: Development and Application of a Pyridylidene-Cu Complex” Yuan Huang and M. Kevin Brown* Angew. Chem. Int. Ed. 2019 58, 6048.
Mechanistic Studies:
To enable the next generation of reaction development, study of mechanism is crucial to our program. In close connection with our experimental efforts we frequently collaborate with computational chemists to provide a comprehensive view of our reactions.
Representative Papers:
1) “Ni-Catalyzed Arylboration of Unactivated Alkenes: Scope and Mechanistic Studies” Stephen R. Sardini, Alison L. Lambright, Grace L. Trammel, Humair M. Omer, Peng Liu,* and M. Kevin Brown* J. Am. Chem. Soc. 2019 141, 9391.
2) “Allenoates in Enantioselective [2+2] Cycloadditions: From a Mechanistic Curiosity to a Stereospecific Transformation” Johannes M. Wiest, Michael L. Conner and M. Kevin Brown* J. Am. Chem. Soc. 2018 140, 15943
Natural Product Synthesis:
With the methods developed in our lab, we often target the synthesis of complex natural product molecules. In addition, en route to the target molecule, additional problems are encountered that frequently require further innovations in methods development.
Representative Papers:
1) “Evolution of a Strategy for the Enantioselective Synthesis of (-)-Cajanusine” Renyu Guo, Brittany P. Witherspoon, and M. Kevin Brown* J. Am. Chem. Soc. 2020, 142, 5002
2) “Lessons in Strain and Stability: An Enantioselective Synthesis of (+)-[5]-Ladderanoic Acid” Erin N. Hancock, Erin L. Kuker, Dear J. Tantillo and M. Kevin Brown* Angew. Chem. Int. Ed. 2020, 59, 436