The discovery and development of small organic molecules as new medicines is one of the most important objectives in modern biomedical research. Central to fueling the pipeline of new medicines is the chemical synthesis of novel small molecules. Stereochemically and topologically complex molecules are particularly challenging to prepare in useful quantities, even with state-of-the-art chemical reactions. Therefore, the discovery and development of novel and widely applicable stereoselective chemical reactions stands at the forefront of modern organic chemistry research.
Methods Development:
Alkenes constitute an appealing class of starting materials for chemical synthesis because of their wide availability and ease of synthesis. Difunctionalization reactions are particularly important as two new bonds and two new stereocenters can be generated in a single operation thus allowing for the rapid buildup of molecular complexity. Along this theme, we are making innovations in transition-metal catalyzed reactions and cycloaddition processes.
Representative Papers:
1) Boron Enabled Directed [2+2]- and Dearomative [4+2]-Cycloadditions Initiated by Energy Transfer. Souvik Adak, Partha Sarathi Hazra, Carter B. Fox, and M. Kevin Brown Angew. Chem. Int. Ed. 2024, e202416215
2) Synthesis of Secondary Boronates via Deaminative Cross-Coupling of Alkyl Nitroso Carbamates and Boronic Acids. Shashwati Paul and M. Kevin Brown, Angew. Chem. Int. Ed. 2024, e202408432
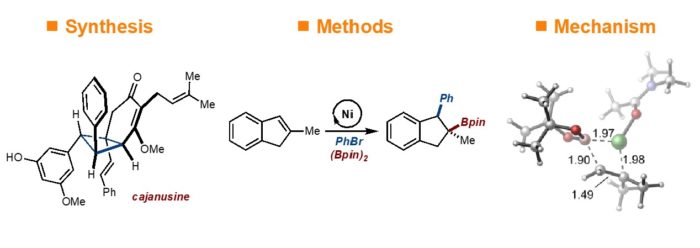
3) Diverse Synthesis of C‑Glycosides by Stereoselective Ni-Catalyzed Carboboration of Glycals. Mao-Yun Lyu, Samuel A. Jacobo, and M. Kevin Brown J. Am. Chem. Soc., 2024, 146, 28, 18866–18872
4) Synthesis of Borylated Carbocycles by [2 + 2]-Cycloadditions and Photo-Ene Reactions. Jarett M. Posz, Neetu Sharma, Paige A. Royalty, Yanyao Liu, Christophe Salome,* Thomas C. Fessard, and M. Kevin Brown* J. Am. Chem. Soc. 2024, 146, 14, 10142–10149
Mechanistic Studies:
To enable the next generation of reaction development, study of mechanism is crucial to our program. In close connection with our experimental efforts we frequently collaborate with computational chemists to provide a comprehensive view of our reactions.
Representative Papers:
1) Photochemical Dearomative Cycloadditions of Quinolines and Alkenes: Scope and Mechanism Studies. Renyu Guo,a Souvik Adak,a Peter Bellotti,b Xinfeng Gao,a W. Walker Smith,a Sam (Ngan) Le,c Jiajia Ma,b K. N. Houk,*,d Frank Glorius,*,b Shuming Chen*,c and M. Kevin Brown*,a J. Am. Chem. Soc. 2022, 144, 17680-17691.
Natural Product Synthesis:
With the methods developed in our lab, we often target the synthesis of complex natural product molecules. In addition, en route to the target molecule, additional problems are encountered that frequently require further innovations in methods development.
Representative Papers:
1) Boronic Ester Enabled [2 + 2]-Cycloadditions by Temporary Coordination: Synthesis of Artochamin J and Piperarborenine B. Yanyao Liu, Dongshun Ni, and M. Kevin Brown, J. Am. Chem. Soc. 2022, 144, 18790–18796.